In the winemaking-process chart below, our first encounter with oxygen is the concern with enzymic oxidation in the must stage of wine production.
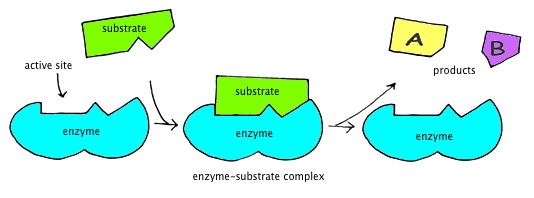
The figure below graphically illustrates the concept of enzymatic oxidation. In the figure, an enzyme binds with a substrate (in the presence of O₂), with the substrate broken into two products as a result of this coupling. In chemical terms, oxygen attracts electrons from other molecules, changing their chemical nature.
 |
| Source (sites.google.com) |
In the case of wine, this oxidation can cause binding of thiol (aroma) compounds and a browning of the color. In the case of wine, the substrate are phenolic compounds. The phenols in grapes are discussed in greater detail below.
Phenols
Phenols are highly reactive chemical compounds of primary importance in the quality of red wines. Phenol, the basic building block, is an aromatic organic compound (formula C6H5OH) where the phenyl group (C6H5, where six bonded carbon atoms with alternating double bonds are connected to five hydrogen atoms) is bonded to a hydroxyl group (OH, where the oxygen atom is covalently bonded to an hydrogen atom). A graphical representation of a phenol is provided below.
Phenolic compounds are:
Table 1. Generalized concentration of various phenolic compounds
present in wine.
Source: Kennedy, et al., Grape and wine phenolics: History and perspective,
AJEV, 57(3), September 2006.
Phenolic compounds are:
- Responsible for the color of red grapes and wine
- Involved in the oxidative browning of white wines
- Contributors to taste and astringency through interactions with salivary proteins
- Another measure of wine quality.
Table 1. Generalized concentration of various phenolic compounds
present in wine.
| Phenolic | White Wine (mg/L) | Light Red Wine (mg/L) | Full Red Wine (mg/L) |
| Volatile | Trace | 10 | 40 |
| Hydroxycinnamic acids | 150 | 200 | 200 |
| Other nonflavonoids | 25 | 40 | 60 |
| Anthocyanins | 0 | 200 | 400 |
| Catechins | 25 | 150 | 200 |
| Polymeric catechins | 0 | 600 | 900 |
| Totals | 200 | 1200 | 1800 |
AJEV, 57(3), September 2006.
Sources of Phenolics: Skins, Seeds, and Stems
The figure below illustrates the flavonoid phenolic compounds in wines and their sources.
 |
| Sources of Phenols (Source: mdpi.com) |
Skin
The berry skin consists of an outer layer with a wax-like coating (cuticle) and 6 to 10 layers of thick-walled cells (hypodermis) which accumulate phenolic compounds in fairly high concentrations as the berry matures (Dharmadhikari, McGlynn). The main components of the skin are phenols, aromatic substances, potassium, and other minerals.
Skin contact increases the amount of hydroxycinnamates, gallic acids, and flavonoids in a wine. Flavonoids increase slightly with contact time but strongly with temperature. These compounds are of concern because they contribute to bitterness and astringency and also serve as substrates for oxidation in white wines. While there are elevated levels of astringency in skin-contact white wines, they are nowhere near as high as in red wines. First, even though tannin is extracted from the skin of the white grape, the lack of anthocyanins means that only tannin-tannin bonds are formed, a combination that is less soluble in alcohol. Second, during fermentation, most of the tannin will precipitate out, thus limiting its ability to negatively impact the wine's sensory characteristics.
Aromatic substances are located in the skin and layers of cells immediately below it. Examples of these compounds include (Dharmadhikari):
- 2-methoxy-3-isobutyl pyrazine -- imparts bell pepper odors to Cabernet Sauvignon and Sauvignon Blanc
- 4-vinylguaiacol and 4-vinylphenol -- spicy, clove-like, and medicinal odors in some Gewurtztraminers
- Terpenes -- can be found in Muscats and Rieslings.
Seeds
Grape seeds are comprised of an outer seed coat, an endosperm, and an embryo, with the seed coat containing relatively large concentrations of tannin. Jackson stipulates that the predominant phenolics in seeds are the flavan-3-ols catechin, epicatechin, and procyanidin polymers (the latter a condensed tannin). The tannins in the seed walls are more polymerized, and contain a higher proportion of epicatechin gallate, than those in the inner portions. Phenol levels in the seed are higher than in the skin or stems (60% versus 20% each) but they are seldom extracted to their "full potential" during wine production due to the lipid coating which retards tannin extraction until alcohol content becomes a facilitator (Jackson).
Seed tannins weigh, on average, 3.5 - 5 mg per berry while skin tannins weigh in between 0.5 and 0.9 mg. Seed tannin polymers are shorter than skin tannin polymers (the longer the tannin chain the higher the astringency) yet seed tannins are perceived as harsher, greener, and more astringent due to a greater degree of galloylation.
Grape tannins accumulate during the first period of berry growth with skin tannin synthesis beginning earlier than seed tannin synthesis and then ending with the conclusion of the first phase of growth. Seed tannin synthesis continues into the early period of berry ripening before concluding. Both skin and seed tannins continue to mature during the berry ripening phase.
Skin tannins release early and easily but then plateau. Seed tannin release is slow, steady, and long and requires alcohol as a solvent. Tannin extraction will continue throughout fermentation with the ratio tilting in favor of seed tannin sometime during the process.
Stems
Grape stems are comprised of cellulose (approximately 30%), hemicellulose (21%), lignin(17%), tannin (16%), proteins (6%), and other constituents. As was the case for seeds, stem flavan-3-ols occur primarily as catechin, epicatechin, epigallocatechin, epicatechin-gallate, and condensed tannins (procyanidin oligomers and polymers).
In a study of wines made with varying levels of stem inclusion, Suriano, et al., came to the following conclusions:
Stem condensed tannins are also considered to be very bitter and astringent and fall between skin and seed tannins in size. Green stems should be avoided as it will take years for the wine in which it is included to mellow out (Pambianchi).
Summary
Grape seeds are comprised of an outer seed coat, an endosperm, and an embryo, with the seed coat containing relatively large concentrations of tannin. Jackson stipulates that the predominant phenolics in seeds are the flavan-3-ols catechin, epicatechin, and procyanidin polymers (the latter a condensed tannin). The tannins in the seed walls are more polymerized, and contain a higher proportion of epicatechin gallate, than those in the inner portions. Phenol levels in the seed are higher than in the skin or stems (60% versus 20% each) but they are seldom extracted to their "full potential" during wine production due to the lipid coating which retards tannin extraction until alcohol content becomes a facilitator (Jackson).
Seed tannins weigh, on average, 3.5 - 5 mg per berry while skin tannins weigh in between 0.5 and 0.9 mg. Seed tannin polymers are shorter than skin tannin polymers (the longer the tannin chain the higher the astringency) yet seed tannins are perceived as harsher, greener, and more astringent due to a greater degree of galloylation.
Grape tannins accumulate during the first period of berry growth with skin tannin synthesis beginning earlier than seed tannin synthesis and then ending with the conclusion of the first phase of growth. Seed tannin synthesis continues into the early period of berry ripening before concluding. Both skin and seed tannins continue to mature during the berry ripening phase.
Skin tannins release early and easily but then plateau. Seed tannin release is slow, steady, and long and requires alcohol as a solvent. Tannin extraction will continue throughout fermentation with the ratio tilting in favor of seed tannin sometime during the process.
Stems
Grape stems are comprised of cellulose (approximately 30%), hemicellulose (21%), lignin(17%), tannin (16%), proteins (6%), and other constituents. As was the case for seeds, stem flavan-3-ols occur primarily as catechin, epicatechin, epigallocatechin, epicatechin-gallate, and condensed tannins (procyanidin oligomers and polymers).
In a study of wines made with varying levels of stem inclusion, Suriano, et al., came to the following conclusions:
- Wines vinified in the presence of stems were higher in tannins, flavans, vanillin, and proanthocyanidins
- Stems conferred more structure and flavor to the wines
- Stems facilitated must aeration thus promoting synthesis of higher alcohols and ethyl esters by the yeast.
Stem condensed tannins are also considered to be very bitter and astringent and fall between skin and seed tannins in size. Green stems should be avoided as it will take years for the wine in which it is included to mellow out (Pambianchi).
Summary
To summarize (Gil, et al.):
- Seeds and stems are major sources of phenolic compounds that condition the final composition of the wine
- Seeds release significant amounts of flavan-3-ol monomers as well as proanthocyanidins with relatively low mean degree of polymerization and a high percentage of galloylation
- Seeds also increase astringency and bitterness and generate a slight but significant decrease in ethanol content, probably through the release of potassium and water
- Stems also release flavan-3-ol monomers and proanthocyanidins but their composition differs from those of the seeds
- (+)-gallocatechin replaces (-)-epicatechin
- Procyanidins had a higher mean degree of polymerization than those from seeds and a higher percentage of prodelphinidins
- Stems significantly increased the pH and decreased the titratable acidity and ethanol content (probably through the solubilization of potassium and water) of the finished wine.
Phenol Characteristics: Tannins
Key tannin properties are:
Flavan-3-ols
Grape-derived tannins are primarily monomers and increase in quantity from fruit set through veraison. It is thought that the primary purpose of these compounds in the plant is as a defense against bacteria, viruses, and higher herbivores. The naturally occuring flavan-3-ol compounds are catechin and epicatechin which register at between 10 and 50 mg/L in white wines and 200 mg/L in reds. Catechin and epicatechin are characterized by a single OH group at position 3 of the C ring (shown below). The formation of the compounds gallocatechin and epigallocatechin is signaled by the presence of three OH groups in the B ring. We can also have a gallic acid acylated at position 3 of the C ring to form catechin-3-o-gallate or epicatechin-3-o-gallate.
Tannins have the ability to associate (form long chains; also called polymerization) and grape tannin polymers are called proanthocyanidins or condensed tannins. These condensed tannins are unstable and, in the acidic wine environment, are subject to polymerization, hydrolysis, and depolymerization. A limited degree of polymerization occurs during fruit maturation.
If a tannin is hydrolyzed under the acidic conditions in wine, it can break up into shorter lengths, producing one electron-neutral and one positively charged tannin. The positively charged tannin thus released will react with another tannin or with an anthocyanin. In the case of tannin-tannin interaction, a longer, non-colored polymer is formed. This tannin polymerization continues until the chain is end-capped by an anthocyanin molecule.
Increasing polymerization brings increased polymer size which is quantified by a measure called degree of polymerization (DP). DP increases with wine age, yielding greater wine suppleness and a reduction in astringency. Tannin quality is generally considered to be a function of the degree of polymerization and the level of association with other molecules.
Hydrolizyable Tannins
According to Puech, et al., hydrolizyable tannins contain a polyhydric alcohol (more than one hydroxyl group) as the basic structural unit of which the hydroxyl group has been esterified by gallic and hexahydroxydiphenic (HHDP) acid. The bonds between these units can be easily broken -- through enzymatic action or contact with an acid or base -- to produce free gallic acid and HHDP acid, the latter of which spontaneously converts into the lactone ellagic acid by internal condensation. Oak-sourced tannins are classified as gallotannins or ellagitannins depending on the type of acid formed.
Ellagitannins may comprise up to 10% of heartwood. In the plant, ellagitannins are toxic to micro-organisms and provide the oakwood with a defense against fungal degradation. It differs from lignin by its ability to bind with, and precipitate, alkaloids, gelatins, and other proteins. Ellagitannins may be monomeric (one glucose core) or oligomeric with differences based on the position of the couplings. The most frequent ellagitannin monomers extracted from oak are vescalagin and castalagin while the most important oligomers are roburin A and E, both vescalagin or castalagin dimers, or granidin. Fully 50% of the total ellagitannin in heartwood is unextractable.
Ellagitannins influence the structure of phenolic compounds and red wines by speeding up the condensation of procyanidins while limiting their oxidative and precipitative degradation.
- Astringency
- Bitterness
- Reaction with ferric chloride
- The ability to bind with protein.
Flavan-3-ols
Grape-derived tannins are primarily monomers and increase in quantity from fruit set through veraison. It is thought that the primary purpose of these compounds in the plant is as a defense against bacteria, viruses, and higher herbivores. The naturally occuring flavan-3-ol compounds are catechin and epicatechin which register at between 10 and 50 mg/L in white wines and 200 mg/L in reds. Catechin and epicatechin are characterized by a single OH group at position 3 of the C ring (shown below). The formation of the compounds gallocatechin and epigallocatechin is signaled by the presence of three OH groups in the B ring. We can also have a gallic acid acylated at position 3 of the C ring to form catechin-3-o-gallate or epicatechin-3-o-gallate.
 |
| Source: ergogenics.de |
Tannins have the ability to associate (form long chains; also called polymerization) and grape tannin polymers are called proanthocyanidins or condensed tannins. These condensed tannins are unstable and, in the acidic wine environment, are subject to polymerization, hydrolysis, and depolymerization. A limited degree of polymerization occurs during fruit maturation.
If a tannin is hydrolyzed under the acidic conditions in wine, it can break up into shorter lengths, producing one electron-neutral and one positively charged tannin. The positively charged tannin thus released will react with another tannin or with an anthocyanin. In the case of tannin-tannin interaction, a longer, non-colored polymer is formed. This tannin polymerization continues until the chain is end-capped by an anthocyanin molecule.
Increasing polymerization brings increased polymer size which is quantified by a measure called degree of polymerization (DP). DP increases with wine age, yielding greater wine suppleness and a reduction in astringency. Tannin quality is generally considered to be a function of the degree of polymerization and the level of association with other molecules.
Hydrolizyable Tannins
According to Puech, et al., hydrolizyable tannins contain a polyhydric alcohol (more than one hydroxyl group) as the basic structural unit of which the hydroxyl group has been esterified by gallic and hexahydroxydiphenic (HHDP) acid. The bonds between these units can be easily broken -- through enzymatic action or contact with an acid or base -- to produce free gallic acid and HHDP acid, the latter of which spontaneously converts into the lactone ellagic acid by internal condensation. Oak-sourced tannins are classified as gallotannins or ellagitannins depending on the type of acid formed.
Ellagitannins may comprise up to 10% of heartwood. In the plant, ellagitannins are toxic to micro-organisms and provide the oakwood with a defense against fungal degradation. It differs from lignin by its ability to bind with, and precipitate, alkaloids, gelatins, and other proteins. Ellagitannins may be monomeric (one glucose core) or oligomeric with differences based on the position of the couplings. The most frequent ellagitannin monomers extracted from oak are vescalagin and castalagin while the most important oligomers are roburin A and E, both vescalagin or castalagin dimers, or granidin. Fully 50% of the total ellagitannin in heartwood is unextractable.
Ellagitannins influence the structure of phenolic compounds and red wines by speeding up the condensation of procyanidins while limiting their oxidative and precipitative degradation.
Phenol Characteristics: Anthocyanins
The most important source of color in red wines is anthocyanin, a class of phenolic compound resident in grape skins. As is the case for all phenolic compounds, anthocyanin is synthesized from the amino acid phenylalanin through the phenylpropanol and flavonoid pathways.
Anthocyanins are synthesized directly from anthocyanidins by glycosylation (adding of a sugar to a protein) at the 3 and 5 positions of the C ring and are accumulated in the berry skins from veraison until full maturity. After synthesis in the cytosol (fluid portion of the cell cytoplasm), anthocyanins are transported into the vacuole (cell organelle responsible for a number of functions including nutrient storage) where they are stored as colored coalescences called anthocyanic vascular inclusions.
Environmental effects can influence anthocyanin content in the fruit but its composition is most closely associated with grape variety. In the fruit, anthocyanin has the following functions (Flamini, et al.):
- Protection against solar exposure and and UV radiation
- Protection against pathogen attacks
- Protection against oxidation attacks
- Protection against attacks by free radicals
- Attracting animals for seed dispersal after the fruit has attained maturity
 |
| Grape anthocyanins. Source: gopixpic.com |
As discussed previously, anthocyanins exist in grapes as glycosides (a bonding of the flavonoid component -- called an anthcyanidin -- with a sugar), a situation which increases both the chemical stability and solubility of the anthocyanidin.
The figure at the top shows the anthocyanidins in native form while the bottom shows a glucosylated Malvidin, Malvidin-3-glucoside.
The anthocyanin can be further complexed through the bonding of the sugar with either acetic, coumaric, or caffeic acid (Jackson).
 |
| Malvidin-3O-coumaroylglucoside |
In wine, spectral color is a function of anthocyanin concentration, the concentration of co-factors which bind with anthocyanin to cause co-pigmentation, and the number and quality of polymeric pigments (Enology Note #120).
In young red wines, anthocyanins occur predominantly as a dynamic equilibrium between one molecular state bonded to sulfur dioxide and four free-form states. Most of these forms are colorless within the range of of wine pH. The exception is a small portion that exists in the flavylium state (2-phenylchromenylium ion), that portion being dependent on pH and free-sulfur dioxide content. According to Jackson, low pH increases the concentration of the flavylium (thus increasing redness) while increases in pH will decrease the color density as the proportion of anthocyanin in the flavylium declines.
When sulfites bind to anthocyanins, the anthocyanins go from a colored to a colorless form (Practical Winery). Because of its effectiveness as an anthocyanin bleacher, sulfur dioxide is the most significant factor affecting the color density of young red wines (Jackson).
The free form of anthocyanin is rendered stable by a number of short- and long-term factors. The short-term factor that is most impactful is co-pigmentation, a process whereby anthocyanin complexes are held together by hydrophobic interaction with non-anthocyanin compounds (anthocyanin-anthocyanin aggregates combined similarly are referred to as self-associations). According to Jackson, the stacking of molecules in these complexes "physically limits water access to (and hydration of) red flavylium and bluish quinoidal base states to colorless carbinol forms." In the case of these co-pigmentation complexes "covalent bonds form between acyl groups of anthocyanin and co-pigments."
The principal compounds which act as co-pigments are epicatechin, procyanidins, cinnamic acids, and hydroxycinnamoyl esters but a wide array of compounds may be involved in this role (Jackson).
Co-pigmentation has the following effects on young red wines (Jackson; Boulton):
- It significantly increases color density and may affect color tint
- It accounts for over half of the observed color of young red wines
- It is important to the purplish coloration of young wines
- Color enhancement has been found to be between two and ten times that expected from the pigment alone
- It also adds temporary stability towards oxidation and SO₂ bleaching.
Phenols Levels in Grapes
The phenol levels in grapes can vary based on a number of factors (Angelosante, Guildsomm):
- The grape variety
- Yields -- excessive yields can result in lower levels of color and tannin
- Growing environment
- Higher elevations mean more sunlight and higher polyphenol levels
- Cooler temperatures prolong the ripening period -- tannins and color ripen at lower sugar levels
- Hillsiide vineyards have less water and higher polyphenol production
- Hydric stress
- Seems to induce the plants to produce more skin tannins
- Smaller berries so higher skin-to-juice ratio
- Excess nitrogen causes the vine to cease production of polyphenols
******************************************************************************************************
I will cover the enzymic oxidation process in my next post.







No comments:
Post a Comment