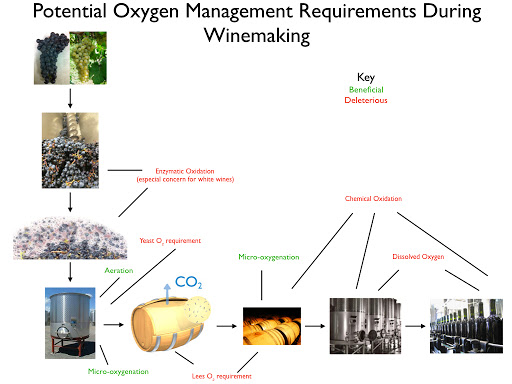
Wine oxidizes when exposed to air via two primary mechanisms: enzymic and non-enzymic oxidation. Enzymic oxidation primarily afflicts wine must and requires the presence of the enzyme Tyrosinase (or Laccase, in the case of botrytized must), phenolic compounds (flavonols, anthocyanins, tannins, etc.), oxygen, and metallic co-factors (iron, copper, etc.). The effects of oxidation on wine are browning, loss of fruity aromas, and aldehydic aromas. I described the process of enzymic oxidation in my most recent post and will advance strategies to avoid/combat its effects in this one.
The chart below provides a graphical overview of the available strategies. I discuss each in the following text.
Oxygen Elimination
Reductive Winemaking
The essence of reductive winemaking is the production of wine without the presence of oxygen. Grapes are harvested from cool regions and the juice is fermented cold in closed stainless steel tanks. Juice is protected, as is the wine through maturation and bottling. This method is particularly beneficial for grape varieties such as Sauvignon Blanc, Petit Manseng, Chenin Blanc, and Gewurtztraminer that are rich in varietal aromas that can be placed at risk in the face of oxidizing effects.
As Lance Cutler (Achieving Balance in Reductive Winemaking, Wine Business) points out, "Keeping wine away from oxygen can create some vibrantly fruity wines, but this same lack of oxygen might encourage the development of reduced sulfur compounds."
As Lance Cutler (Achieving Balance in Reductive Winemaking, Wine Business) points out, "Keeping wine away from oxygen can create some vibrantly fruity wines, but this same lack of oxygen might encourage the development of reduced sulfur compounds."
Ascorbic Acid
When used in combination with sulfur dioxide, ascorbic acid can be a powerful tool to utilize the dissolved oxygen available in the juice and inhibit aromatic oxidation. It can also delay the onset of browning by converting quinones back to the original phenol. The by-products of the ascorbic acid reactions are dehyroascorbic acid and hydrogen peroxide, the latter of which is itself a strong oxidant.
Ascorbic acid converts oxygen to hydrogen peroxide 1700 times faster than sulfur dioxide alone but the sulfur dioxide is needed to quickly scavenge the hydrogen peroxide. The dehyroascorbic acid undergoes rapid degradation into a variety of species "including numerous carboxylic acids, ketones, and aldehydes."
The recommended levels of ascorbic acid addition range between 50 and 200 mg/L. The use of ascorbic acid is somewhat controversial as it tends to produce fruitier wines and is thought to increase the potential for oxidation in the longer term.
Tannins
Tannins offer some antioxidant properties in that they can (i) bind to oxygen directly, (ii) block enzymatic oxidases (prevent them from working), and (iii) inhibit the radicals that contribute to many oxidative processes.
Substrate Elimination
Hyperoxidation
The deliberate introduction of oxygen into the juice causes enzymatic oxidation of the phenols. The process entails spraying the juice in the tank with oxygen for about 30 minutes and then racking the juice from tank to tank a few times. Alternatively, oxygen can be used in lieu of nitrogen when using flotation to clarify the juice, allowing it to settle, and then racking it from the brown precipitate just prior to fermentation. This oxidation will cause browning of the juice but the phenols will have been polymerized and will precipitate out.
Clarification is required to reduce the suspended solids to less than 1% by weight in order to remove the major part of the phenolic precipitate. This clarification must be completed before fermentation begins as the precipitate will re-dissolve in alcohol. The clarified juice will retain a brown color but this residual browning will be eliminated by the reducing conditions of alcoholic fermentation and absorption by yeasts (Schneider, Hyperoxidation: A Review, AJEV, 1998).
The brown pigment absorbed by the yeasts during alcoholic fermentation will fall to the bottom of the tank with the lees and can be removed in a post-fermentation racking. Fining and/or filtration can be utilized for additional clarification if required.This process renders the wine less susceptible to in-bottle browning (due to the elimination of the phenols) as well as reduces bitterness in the wine.
Hyperoxidation requires that SO2 additions be withheld from the must as the oxidative enzymes are inhibited in its presence. For example, tyrosinase registers a 90% decrease in activity when 50 mg/L of SO is added to the must. SO2 also reduces caftaric acid quinone and enhances the solubility of phenolic molecules. These effects will limit the extent and effectiveness of the hyperoxidation.
The deliberate introduction of oxygen into the juice causes enzymatic oxidation of the phenols. The process entails spraying the juice in the tank with oxygen for about 30 minutes and then racking the juice from tank to tank a few times. Alternatively, oxygen can be used in lieu of nitrogen when using flotation to clarify the juice, allowing it to settle, and then racking it from the brown precipitate just prior to fermentation. This oxidation will cause browning of the juice but the phenols will have been polymerized and will precipitate out.
Clarification is required to reduce the suspended solids to less than 1% by weight in order to remove the major part of the phenolic precipitate. This clarification must be completed before fermentation begins as the precipitate will re-dissolve in alcohol. The clarified juice will retain a brown color but this residual browning will be eliminated by the reducing conditions of alcoholic fermentation and absorption by yeasts (Schneider, Hyperoxidation: A Review, AJEV, 1998).
The brown pigment absorbed by the yeasts during alcoholic fermentation will fall to the bottom of the tank with the lees and can be removed in a post-fermentation racking. Fining and/or filtration can be utilized for additional clarification if required.This process renders the wine less susceptible to in-bottle browning (due to the elimination of the phenols) as well as reduces bitterness in the wine.
Hyperoxidation requires that SO2 additions be withheld from the must as the oxidative enzymes are inhibited in its presence. For example, tyrosinase registers a 90% decrease in activity when 50 mg/L of SO is added to the must. SO2 also reduces caftaric acid quinone and enhances the solubility of phenolic molecules. These effects will limit the extent and effectiveness of the hyperoxidation.
Hyperoxidation, then, uses the strength of oxidation in the early stages of winemaking to neuter the substrate in the early stages of winemaking and prevent it from becoming an oxidation resource in the bottle.
White juice that was not exposed to skin contact can be saturated once with oxygen and that should be sufficient to remove most phenolic compounds. If there has been skin contact, the juice can be left for 30 minutes after saturation and then be sparged for an additional 30 minutes.
Fining
Phenolic molecules can be removed from the must and wine by fining with gelatin, PVPP, bentonite, or activated charcoal. The latter should be handled with care as it can strip the flavor compounds from the wine if the dosage is too high.
Enzyme Elimination
Sulfur Dioxide
Sulfur dioxide is the most important and widely used chemical in the battle against oxidative browning. It is a colorless, non-flammable gas which exists in wine both in the dissolved molecular state as well as ionized bisulfate and sulfite. It is a normal wine constituent -- a by-product of yeast metabolism -- levels of which (depending, primarily, on yeast strain and fermentation conditions) can range between 10 and 100 mg/L, but is usually around 30 mg/L. Because of toxicity at high levels of use, the levels in wine are regulated with the US stipulating levels at or below 350 mg/L and the French 150 mg/L for red wines and 200 mg/L for whites.
Sulfur dioxide is the most important and widely used chemical in the battle against oxidative browning. It is a colorless, non-flammable gas which exists in wine both in the dissolved molecular state as well as ionized bisulfate and sulfite. It is a normal wine constituent -- a by-product of yeast metabolism -- levels of which (depending, primarily, on yeast strain and fermentation conditions) can range between 10 and 100 mg/L, but is usually around 30 mg/L. Because of toxicity at high levels of use, the levels in wine are regulated with the US stipulating levels at or below 350 mg/L and the French 150 mg/L for red wines and 200 mg/L for whites.
Sulfur dioxide can be found in juice and wine in both free and bound forms. In juice, the molecule binds to sugars, grape skins, grape solids, and laccase (catalyzing enzyme found in botrytized grapes) compounds while, in wine, it binds to acetaldehyde, keto acids, glucose, quinones, and monomeric anthocyanins. The total sulfur dioxide in a wine is the sum of free plus bound fractions with the bound component being unavailable for "beneficial" activities.The distribution and characteristics of free sulfur dioxide in wine is presented in Table 1 below.
| Free Sulfur Dioxide State | Characteristics |
| Molecular (SO₂) | - Exists as gas or single molecules dissolved in wine - Most important antimicrobial form of SO₂ - Can act indirectly as an antioxidant - Sensorially detectable |
| Bisulfite (HSO₃) | - Predominant form of free SO₂at wine and juice pH values (3 - 4) - Binds with many wine components - Prevents enzymatic oxidation - Insignificant as an antimicrobial |
| Sulfite (SO₃) | - Small quantities - Not a very effective antioxidant in wine |
Boulton has reported that the addition of sulfur dioxide at levels between 25 and 75 mg/L will reduce tyrosinase activity between 75% and 97%. This inhibition may be the result of a binding of a sulfhydril group on the enzyme or a bisulfite inhibition resulting from the interaction of sulfites and intermediate quinones.
Sulfur dioxide can reverse the effects of oxidized quinones to colorless phenols. Sulfites react slowly with oxygen, but react with important intermediates (hydrogen peroxide, acetaldehyde, quinones).
Pasteurization
Laccase comes into the winemaking environment on the back of Botrytis-cinerea-infected fruit. Sulfur dioxide does not significantly impact the operation of laccase. For example, the addition of 150 mg/L sulphur dioxide yields only a 20% reduction in laccase oxidative activity; as a matter of fact, sulfur dioxide can serve as a substrate in the oxidation process.
The Australian Wine Research Institute (AWRI) recommends testing for laccase activity as early as possible and, if detected, heat treatment (pasteurization) should be considered to deactivate the enzyme prior to fermentation. The application of 70℃ heat for 30 seconds is sufficient to destroy laccase activity.
Laccase requires oxygen to cause oxidative damage therefore the winemaker should employ techniques which reduce must, juice, and wine contact with oxygen. Recommended strategies for handling white grapes include (AWRI):
- Whole-bunch press with a CO₂ cover
- Add pectic enzyme at the higher end of the recommended range and cold soak at low temperature to achieve rapid settling
- Rack and discard the heavy lees
- Trial bentonite additives (starting rate of 0.5 - 1 g/L) to remove moldy characters and settle for 24 hours.
For red grape handling (AWRI):
- Minimize the time between crushing and yeast inoculation and avoid cold soaking
- Laccase is an enzyme and, as such, a protein, which can be fined out using tannin. Use 200 - 500 mg/L of enological tannin at crushing to bind the laccase.
Reducing Agent
Glutathione
Glutathione is a grape- and yeast-produced tripeptide which contains three amino acids: glutamate, cystine, and glycone. It is generally found in must, yeast and wine in its reduced (GSH) or oxidized (GSST) forms, the latter of which contains two molecules of glutathione linked by a sulfide bridge.
Glutathione is important in the wine space, firstly, because of its ability to scavenge ortho-quinones, the main culprit in oxidative browning. The compound plays a critical role in preventing the oxidation of phenols in must by reacting with caftaric ortho-quinones to generate Grape Reduction Product, a stable, colorless compound. The conversion of the oxidized quinone to GRP limits the browning of the juice to some extent (duToit and Kritzinger).
The GSH form of glutathione can also compete with several aromatic compounds for ortho-quinones, thus protecting and preserving certain wine aromas.
Minimizing the loss of glutathione is one of the keys in white winemaking as there is a strong correlation between the concentration of glutathione in the must and the concentration in the young wine and a positive correlation between glutathione in the wine and the wines freshness and longevity.
************************************************************************************************************
The next post in this series will explore the non-enzymatic (chemical) oxidation of wine.